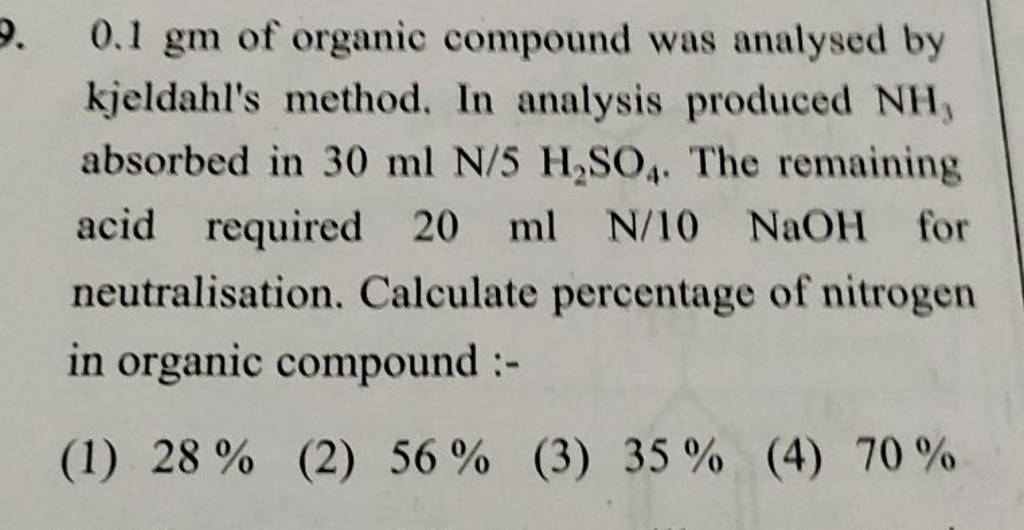
8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N/10 NaOH
Por um escritor misterioso
Descrição
Click here:point_up_2:to get an answer to your question :writing_hand:84 a c01 gm of organic compound was analysed bykjeldahls method in analysis produced nhabsorbed
An organic compound on analysis gave C=48gm,H=8gm and N =56gm. Volume of ..

Nitrogen - ScienceDirect


The Titration in the Kjeldahl Method of Nitrogen Determination: Base or Acid as Titrant?


Practical Organic Chemistry: 1. Purification, PDF, Chromatography

0.35 g of an organic substance was Kjeldahlised and the ammonia obtained was passed into 100ml of M/10H_2SO_4 the excess acid required 154ml of M/10NaOH neutralisation calculate the % of nitrogen in

The Titration in the Kjeldahl Method of Nitrogen Determination: Base or Acid as Titrant?

Analytical Methods for Atomic Absorption Spectroscopy - La Salle

8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N /10 NaOH
de
por adulto (o preço varia de acordo com o tamanho do grupo)







